Wholesale China Peach Ceramide Suppliers Factories - PromaCare-TA / Tranexamic Acid – Uniproma Detail:
Product Paramete
| Trade name | PromaCare-TA |
| CAS | 1197-18-8 |
| Product Name | Tranexamic Acid |
| Chemical Structure | 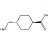 |
| Application | Medicine |
| Package | 25kgs net per drum |
| Appearance | White or almost white, crystalline power |
| Assay | 99.0-101.0% |
| Solubility | Water soluble |
| Shelf life | 4 years |
| Storage | Keep container tightly closed and in a cool place. Keep away from heat. |
Application
Tranexamic Acid, also known as clotting acid, is an antifibrinolytic amino acid, which is one of the commonly used anticoagulants in clinic
This product can be used for:
1. Trauma or surgical bleeding of prostate, urethra, lung, brain, uterus, adrenal gland, thyroid, liver and other organs rich in plasminogen activator.
2. They are used as thrombolytic agents, such as tissue plasminogen activator (t-PA), streptokinase and urokinase antagonist.
3. Induced abortion, placental exfoliation, stillbirth and amniotic fluid embolism caused by fibrinolytic bleeding.
4. Menorrhagia, anterior chamber hemorrhage and severe epistaxis with increased local fibrinolysis.
5. It is used to prevent or reduce bleeding after tooth extraction or oral surgery in hemophilic patients with factor VIII or factor IX deficiency.
6. This product is superior to other antifibrinolytic drugs in hemostasis of mild hemorrhage caused by rupture of central aneurysm, such as subarachnoid hemorrhage and intracranial aneurysm hemorrhage. However, attention must be paid to the risk of cerebral edema or cerebral infarction. As for severe patients with surgical indications, this product can only be used as an adjuvant.
7. For the treatment of hereditary vascular edema, can reduce the number of attacks and severity.
8. Patients with hemophilia have active bleeding.
9. It has definite curative effect on chloasma.
Product detail pictures:

Related Product Guide:
Our organization promises all customers with the first-class products and solutions and the most satisfying post-sale service. We warmly welcome our regular and new clients to join us for Wholesale China Peach Ceramide Suppliers Factories - PromaCare-TA / Tranexamic Acid – Uniproma, The product will supply to all over the world, such as: Jordan, Turin, Orlando, Our tenet is "integrity first, quality best". Now we have confidence in providing you with excellent service and ideal merchandise. We sincerely hope we can establish win-win business cooperation with you in the future!
The sales manager is very patient, we communicated about three days before we decided to cooperate, finally, we are very satisfied with this cooperation!
-

Wholesale China Carbopol 940 Polymer Manufactur...
-
Wholesale China Mintop Minoxidil 5 Factory Supp...
-

Wholesale China Carbomer Safe Quotes Manufactur...
-

China Wholesale Maui Tanning Manufacturers Pric...
-

China Wholesale Lighten Bikini Area Manufacture...
-

Wholesale China Glutathione Benefits Suppliers ...